Hyaluronic Acid Injection
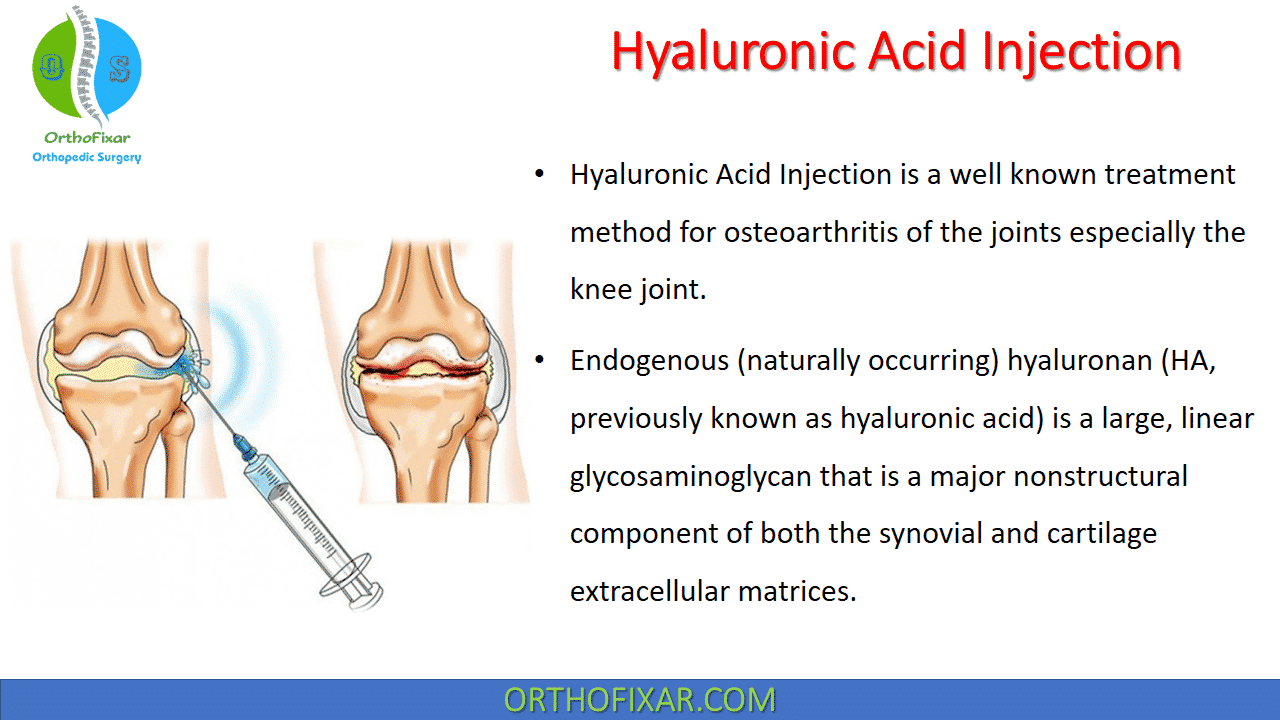
Hyaluronic Acid Injection is a well known treatment method for osteoarthritis of the joints especially the knee joint.
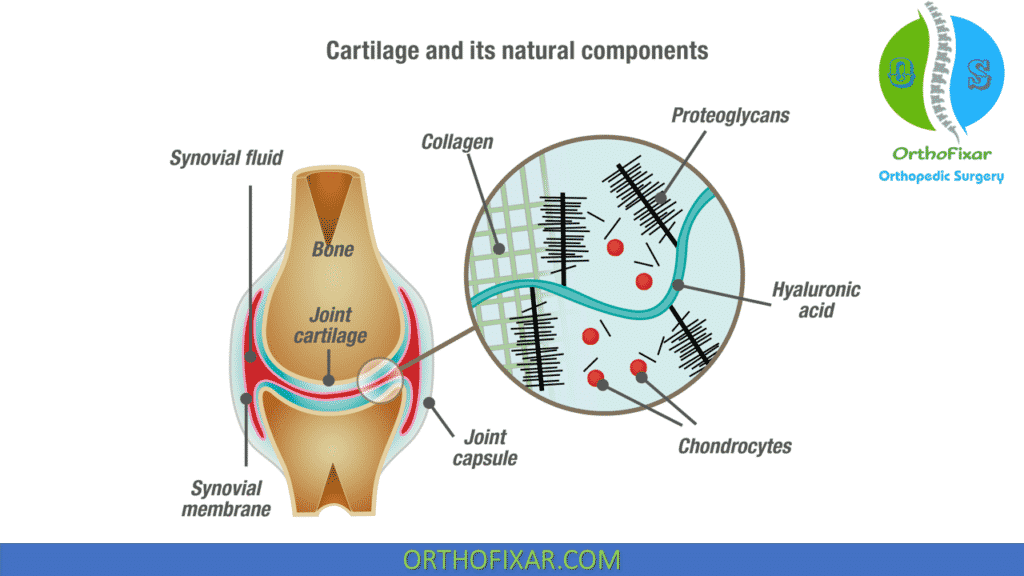
Endogenous (naturally occurring) hyaluronan (HA, previously known as hyaluronic acid) is a large, linear glycosaminoglycan that is a major nonstructural component of both the synovial and cartilage extracellular matrices.
See Also: Anatomic sources of pain in osteoarthritis
Hyaluronic Acid Functions
It is also found in synovial fluid and is produced by the lining layer cells of the joint. These molecules acts as:
- A highly viscoelastic solution that is a viscous lubricant at low shear (during slow movement of the joint – e.g., walking)
- An elastic shock absorber at high shear (during rapid movement – e.g., running).
- As well as conferring viscoelasticity, the other key functions of HA in the joint are lubrication and the maintenance of tissue hydration and protein homeostasis through the prevention of large fluid movements by functioning as an osmotic buffer.
- HA is also considered a physiological factor in the trophic status of cartilage.
In osteoarthritic joints, the capacity of synovial fluid to lubricate and absorb shock is typically reduced. This is partly because of the production of abnormal HA, with a reduction in the size and concentration of the molecules naturally present in the synovial fluid.
Hyaluronic Acid Synthetic
Synthetic HA was isolated from roosters’ combs and umbilical cord tissue and developed for clinical use in ophthalmic surgery and arthritis in the 1960s. The rationale for joint injection therapy was to replace the normal physiological properties lost to the osteoarthritic joint as a consequence of the associated reduction in the volume and quality of HA, a concept known as viscosupplementation injections.
Commercial preparations of HA have the same structure as endogenous HA, although cross-linked HA molecules (known as hylans) were later engineered by molecular linkage to obtain greater elastoviscosity and intraarticular dwell time.
Hyaluronic Acid Injection for Knee Osteoarthritis
Osteoarthritic knees may be treated by the intraarticular injection of HA, usually after any effusion is drained.
The mode of action of exogenous (synthetic) HA and its derivatives is not clear, particularly when an effusion containing endogenous HA is removed and immediately replaced by exogenous HA, which then stays in the joint cavity for only a few days at most.
Perhaps these injections stimulate the synthesis of better-quality, more physiologically normal endogenous HA and/or reduce inflammation. Given the relatively short intraarticular residency, any hypothesis for the mechanism of action must account for the long duration of clinical efficacy that has been reported.
A number of commercial preparations are available for injection , but there is no evidence that any one preparation is superior. The licensed commercial formulations that have been available the longest in the United Kingdom are Hyalgan (sodium hyaluronate) and Synvisc (hylan G-F 20):
- Hyalgan has a lower molecular weight and is licensed as a medicinal product; it is injected once weekly for 5 weeks and is repeatable at no more than 6-month intervals.
- Synvisc has a higher molecular weight and is licensed as a medical device; it is injected once weekly for 3 weeks, repeatable once within 6 months, with at least 4 weeks between courses.
There is also Durolane knee injection, Euflexxa, Fermathron, Orthovisc, Ostenil, Suplasyn and Synocrom.



Hyaluronic Acid Injection Efficacy
The research evidence on the efficacy of HAs is often difficult to interpret because of confounders, including different molecular weights, different injection schedules (ranging from once to a series of five injections) and, despite large numbers of studies, generally poor trial design (e.g., lack of intention to treat analyses and limitations in blinding).
Intraarticular Hyaluronic Acid injection for knee osteoarthritis has been endorsed by two authoritative guidelines, but was rejected by the UK National Institute for Health and Clinical Excellence (NICE) on the grounds of cost. The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) treatment algorithm has recommended intraarticular Hyaluronic Acid for knee osteoarthritis as second-line treatment in patients who remain symptomatic, despite use of nonsteroidal antiinflammatory drugs.
Based on evidence from real-life setting trials and surveys, HA is recommended as a safe and effective component of the multimodal management of knee osteoarthritis (OA).
A systematic review and meta analysis has concluded that from baseline to week 4, intraarticular corticosteroids appear to be relatively more effective for pain than intraarticular Hyaluronan Injections. By week 4, both therapies have equal efficacy but, beyond week 8, HA has greater efficacy.
A Cochrane review has also suggested that the pain relief with HA therapy is achieved more slowly than with steroid injections, but the effect may be more prolonged. Another meta analysis of high-quality trials of intraarticular HA versus placebo has shown that this provides a moderate but real benefit for patients with knee OA.
Two prospective double-blind, randomized, placebo-controlled trials with large numbers of osteoarthritic knee patients have reached opposing conclusions. One study of five weekly injections of Hyalgan versus saline after 1 year showed no treatment effect in any outcome measure. The other study compared a single injection of Synvisc with placebo and, after 6 months, showed clinically relevant pain relief.
Treatment with three weekly injections of intermediate-molecular-weight HA may be superior to low-molecular-weight Hyaluronic Acid injection for knee osteoarthritis symptoms over 6 months, with similar safety. Overall, the evidence suggests that HA and hylan derivatives are superior to placebo in terms of pain reduction, efficacy and quality of life outcomes in patients with osteoarthritic knees, although the effect size is generally small.
Given this, and the cost of these therapies, together with the increased number of clinician visits required, NICE has concluded that the benefits of HA injection therapy would have to be three to five times higher than current estimates before efficacy reaches the standard threshold for cost-effectiveness to the National Health Service (NHS).
NICE also concluded that clinical trials do not suggest that there are subgroups of patients who may have greater benefit from HA treatment, which might improve cost-effectiveness. Limited data are available concerning the effectiveness of multiple courses of HA therapy.
Patients older than 65 years and those with the most advanced radiographic stage of osteoarthritis are less likely to benefit.
Hyaluronic Acid Injection for Hip Osteoarthritis
Some commercial HAs are licensed for use in the hip joint.
No significant differences between HA and placebo were reported by a trial evaluating efficacy and function outcomes in patients with hip osteoarthritis; one systematic review has noted methodological limitations in the literature, which were mainly the absence of a control group in most of the studies, overly short follow-up periods and different ways of measuring outcomes. The review concluded that Hyaluronic Acid Injection for Hip Osteoarthritis should only be used under careful supervision and only in those patients for whom other treatments have failed.
A second systematic review has concluded that despite the relatively low level of evidence of the included studies, HA injection performed under fluoroscopic or ultrasound guidance seems to be effective and appears to be safe and well tolerated, but cannot be recommended as standard therapy in the wider population.
A third review has concluded that this therapy seems to be a valuable technique that may delay the need for surgical intervention, with no difference among products, but further studies are necessary.
The use of HA injections in other joints is being investigated. Encouraging, but inconclusive results have been observed for the treatment of shoulder, carpometacarpal and ankle osteoarthritis.
See Also: Hip Joint Osteoarthritis
HA Injection Safety
The toxicity of intraarticular HA appears to be negligible. No major safety issues have been identified when compared with placebo, but a definitive conclusion is precluded because of sample size restrictions.
HA injections may cause a short-term increase in knee inflammation. A small percentage of patients may experience a transient mild to moderate increase in pain following injection, and some have a flare with marked effusion.
Local reactions to hylan G-F-20 occur more often in patients who have undergone more than one course of treatment. Following corticosteroid injection, these reactions abate without apparent sequelae. As with any injection procedure, there is a very small risk of infection.
The synergistic combination of corticosteroid and HA for simultaneous injection is an approach that has been investigated in a small number of studies. COR1.1, a 560-patient, international, multicenter, randomized, double-blind study has evaluated the safety and efficacy of Hydros-TA (hyaluronic acid that entraps a low-dose corticosteroid, triamcinolone acetonide [TA], 10 mg) versus the HA component alone and the TA component alone. Hydros-TA met the first of its two primary endpoints, demonstrating a statistically significant improvement from baseline in the WOMAC (Western Ontario and McMaster Universities Osteoarthritis) pain score at week 2 versus Hydros-TA.
See Also: WOMAC Score
In addition, Hydros-TA maintained a significant reduction in pain from baseline over 26 weeks. However, patients in the TA component arm continued to show an unexpected significant reduction in pain through 26 weeks. Given the comparable effectiveness at 26 weeks, COR1.1 did not meet its second primary endpoint. Hydros-TA was generally well tolerated, with no treatment-related serious adverse events.
A metaanalysis has indicated that intraarticular HA is an effective and safe alternative therapy for the rheumatoid knee. In another rheumatoid arthritis study, HA and corticosteroid injections generally had similar efficacy rates when patient-perceived satisfaction was used as an index.
References
- National Institute for Health and Care Excellence. https:// www.nice.org.uk/guidance/cg177
- Uthman I, Raynauld JP, Haraoui B. Intra-articular therapy in osteoarthritis. Postgrad Med J. 2003;79:449–453.
- Hyaluronan or hylans for knee osteoarthritis? Drug Ther Bull. 1999;37(9):71–72.
- Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
- Jordan KM, Arden NK, Doherty M, et al. Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT. EULAR Recommendations 2003: an evidence- based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145–1155.
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–1915.
- Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(suppl): S28–S33.
- Bannuru RR, Natov NS, Obadan IE, et al. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–1711.
- Richette P, Chevalier X, Ea HK, et al. Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias. RMD Open. 2015;1(1):e000071.
- Jorgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis. 2010;69:1097–1102.
- Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:113–119.
- Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71:1454–1460.
- Kotz R, Kolarz G. Intra-articular hyaluronic acid: duration of effect and results of repeated treatment cycles. Am J Orthop. 1999;29(suppl 11): 5–7.
- Wang CT, Lin J, Chang CJ, et al. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee; a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86:538–545.
- Qvistgaard E, Christensen R, Torp PS, et al. Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14(2):163–170.
- Fernandez-Lopez JC, Ruano-Ravina A. Efficacy and safety of intraarticular hyaluronic acid in the treatment of hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2006;14(12):1306–1311.
- van den Bekerom MP, Lamme B, Sermon A, et al. What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature. Arch Orthop Trauma Surg. 2008;128(8):815–823.
- van den Bekerom MPJ, Rys B, Mulier M. Viscosupplementation in the hip: evaluation of hyaluronic acid formulations. Arch Orthop Trauma Surg. 2008;128(3):275–280.
- Tagliafico A, Serafini G, Sconfienza LM, et al. Ultrasound-guided viscosupplementation of subacromial space in elderly patients with cuff tear arthropathy using a high weight hyaluronic acid: prospective open-label non-randomized trial. Eur Radiol. 2011;21(1): 182–187.
- Mei-Dan O, Kish B, Shabat S. Treatment of osteoarthritis of the ankle by intra-articular injections of hyaluronic acid: a prospective study. J Am Podiatr Med Assoc. 2010;100(2):93–100.
- Abate M, Pulcini D, Di Iorio A, et al. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des. 2010;16(6):631–640.
- Bernardeau C, Bucki B, Liote F. Acute arthritis after intra-articular hyaluronate injection: onset of effusions without crystal. Ann Rheum Dis. 2001;60:518–520.
- Leopold SS, Warme WJ, Pettis PD, et al. Increased frequency of acute local reaction to intra-articular hylan GF-20 (Synvisc) in patients receiving more than one course of treatment. J Bone Joint Surg Am. 2002;84:1619–1623.
- Rovetta G, Monteforte P. Intraarticular injection of sodium hyaluronate plus steroid versus steroid in adhesive capsulitis of the shoulder. Int J Tissue React. 1998;20(4):125–130.
- Callegari L, Spano E, Bini A, et al. Ultrasound-guided injection of a corticosteroid and hyaluronic acid: a potential new approach to the treatment of trigger finger. Drugs RD. 2011;11(12):137–145.
- http://www.carbylan.com/u/files/cor11_clinical_trial_hydrosta.pdf
- Saito S, Momohara S, Taniguchi A, et al. The intra-articular efficacy of hyaluronate injections in the treatment of rheumatoid arthritis. Mod Rheumatol. 2009;19(6):643–651.
- Lifetime product updates
- Install on one device
- Lifetime product support
- Lifetime product updates
- Install on one device
- Lifetime product support
- Lifetime product updates
- Install on one device
- Lifetime product support
- Lifetime product updates
- Install on one device
- Lifetime product support